Background: CHIP is more commonly encountered in cancer patients, such as those with lymphoma, than in the general population likely attributable to age, cancer risk factors and treatment. While the adverse health associations with clonal hematopoiesis of indeterminate potential (CHIP) are established, its impact on the immediate tolerability of chemotherapy and longer-term blood count reconstitution are unknown. Also, while it is known that certain CHIP drivers, especially those affecting DNA damage responses, may be selected during cancer treatment, comprehensive studies of CHIP clonal dynamics along the course of treatment are lacking. We hypothesized that CHIP may be a risk factor for chemotherapy complications in older lymphoma patients related to dysfunctional bone marrow and blood cells. Our Aims were to ascertain CHIP status using targeted sequencing, investigate the influence of CHIP on adverse events during chemotherapy, and the changes in clonal dynamics.
Methods: We evaluated the prevalence, associations, clonal dynamics and impact of CHIP on treatment toxicity and blood count recovery in lymphoma patients aged > 60 undergoing 1 st or second line cytotoxic chemotherapy. CHIP (Ion torrent NGS, 53 genes including coverage for DNA damage response (DDR) mutations: TP53, PPM1D, CHEK2 and SRCAP was performed on peripheral blood (PB) samples at baseline, after 3 chemotherapy cycles, at 6 and 12 months post chemotherapy. Ion Torrent VAF cutoff was 2%. Integrated genomics viewer (IGV) was used to detect known variants at < 2% VAF. We compared blood count parameters and treatment complications between those with and without CHIP overall or DDR mutations in particular.
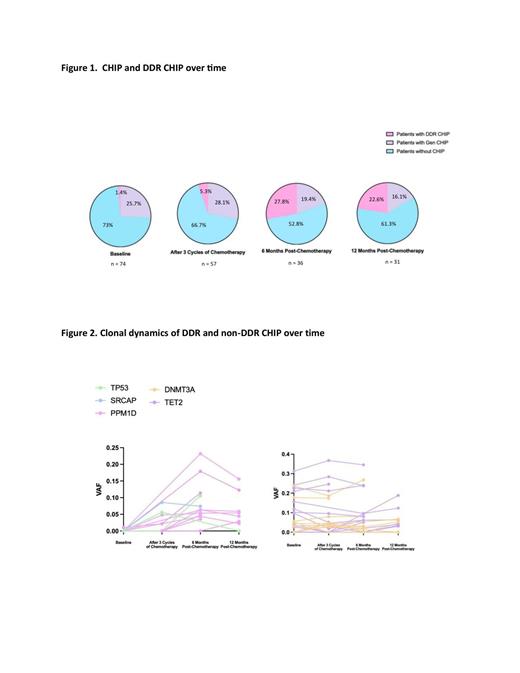
Results: 78 of planned 180 patients are available for analysis and 74 with non-lymphoma-related mutations are included. Median age was 70, 58% female. 61% had DLBCL and 19% follicular lymphoma. 88% received first line chemotherapy (68% anthracycline containing) and 15% had received radiation in the past. With a median follow up of 19 months, 82% remain alive. All CHIP (and DDR) mutations were detected in 27% (1.4%) patients at baseline, in 33.3% (5.3%) after cycle 3, in 47.2% (27.8%) at 6 months and 38.7% (22.6%) 12 months post chemotherapy respectively (Figure 1). IGV inspection and deep sequencing confirmed that most DDR variants were present at <2% VAF at baseline and expanded with treatment. Moreover, DDR CHIP peaked at the 6 months post-chemotherapy timepoint, with contraction in overall prevalence and VAF by 12 months. In contrast, most non-DDR mutations remained stable or increased slightly. The clonal dynamics of CHIP and DDR mutations are visible in Figure 2.
Comparing those, with or without baseline or acquired CHIP or DDR mutations, there were no significant differences in most baseline clinical and laboratory characteristics, chemotherapy dose delays or reductions. In particular, MCV and RDW did not differ at baseline or increase preferentially in those with or without mutations. However, patients with DDR mutations had higher eosinophil counts at baseline (0.2 versus 0.1, p=.01), higher eosinophil counts over time (p=.0003), were more likely to visit an emergency department (86% versus 52%, p=.03), and sustain infections on treatment (43% versus 20% p=.07). A higher percentage of patients with DDR mutations had monocyte counts that exceeded normal reference ranges 6 months post (23% versus 10%, p=.01) and (trend) 12 months post (30% versus 12.9%, p=0.3). More patients with CHIP had marginal zone lymphoma (21% versus 2.5%, p=.04), experienced fewer neurologic toxicities (22% versus 52%, p=.006) but more respiratory infections (19.4% versus 2.3%, p=.02). The monocytes of patients with CHIP (compared without) surpassed baseline monocyte counts at 6 months (57.1% versus 16%, p=.004), and 12 months (52.3% versus 20%, p=0.03) and exceeded normal reference range (21% versus 4%, p=.04) at 6 months.
Conclusion: The prevalence of CHIP is high in older lymphoma patients, with preferential expansion of clones harboring DDR mutations during treatment. Most of these DDR variants would fall below routine clinical NGS reporting thresholds if sampled at baseline, or after post-therapy contraction. DDR CHIP and CHIP overall associate with different complication rates on chemotherapy and both associate with post-treatment monocytosis. These novel DDR CHIP immune alterations warrant further mechanistic study.
Disclosures
Buckstein:Abbvie: Honoraria; Taiho: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Berinstein:Astra Zeneca: Other: Scientific Advisor, Research Funding. Tsui:LifeLabs: Consultancy; Precision Dx: Consultancy; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal